
Secure Boot and Firmware Protection in Embedded Systems
Users of embedded devices – from industrial controllers to consumer electronics – are often unaware of hidden vulnerabilities that threaten […]
Today’s medicine would not be in the same place if software-based solutions were not implemented into it. I’d be tempted to say that most innovations include at least one software element. At Scythe Studio, we’ve worked on projects such as a 3D printer using biological material, a device for robotic spinal surgery, and a home blood diagnostics application. What to classify and how to classify it? It is not at all that obvious.
In this article, I would like to explain the two main types of medical software clearly. The division discussed is in line with the IMDRF – International Medical Device Regulators Forum.
This article is a result of our experience in medical device software development. For years we have participated in the development of modern SiMD and SaMD projects. A combination of expertise in advanced software and embedded hardware techniques with a rich portfolio and ISO 9001:2015 and ISO 13485:2016 certifications proves that we match the highest quality criteria for a medical software development partnership. Check out our services offer.
Software as a Medical Device, commonly referred to as SaMD, represents a distinct category of medical software solutions. Defined by the International Medical Device Regulators Forum (IMDRF), SaMD is software that functions as a medical product in its own right, without needing to be part of a physical medical device or communicate with such.
This classification emphasizes the software’s primary role in the diagnosis, prevention, monitoring, or treatment of diseases and conditions. Software as a medical device means software intended to operate independently.
Typically distributed as applications for desktop or mobile platforms, SaMD allows healthcare providers and patients to access medical functionality directly through their devices. Popular operating systems such as Windows and macOS for desktops, and iOS and Android for smartphones, are common platforms for these applications.
To make it easier for us to visualize this type of software let’s list some examples of SaMD-type applications:
Diagnostic software serves a crucial role in the healthcare sector by supporting medical professionals in the analysis and interpretation of medical images from X-rays, CT scans, and MRIs. This type of SaMD, intended for medical purposes such as accurate diagnostics, helps ensure patient safety by facilitating quicker, more precise assessments.
We have another blog post – “How to develop DICOM medical imaging software?” that you may find interesting. You’ll find there a great explanation of steps to take to introduce the DICOM viewer to a new or an existing piece of software.
Telemedicine software exemplifies SaMD by enabling remote medical consultations and treatments, connecting patients with healthcare professionals via digital platforms. Often in an automated or AI-supported way.
This technology not only expands access to medical care but also enhances patient safety by reducing the need for physical visits. As a medical device, telemedicine software and any software as a medical device project must comply with medical device regulatory, ensuring it meets the necessary standards for security and efficacy in the medical sector.
Health monitoring software, a type of SaMD, operates independently by analyzing user-entered health data such as weight, blood pressure, and other vital parameters. These applications provide valuable insights into a user’s health trends, facilitating proactive healthcare and medical intervention.
Unlike devices that measure parameters directly, this software relies on input data to perform its functions, ensuring compliance with medical device regulations for patient safety and efficacy in the health care.
Therapeutic applications, a specialized form of SaMD, deliver medical interventions directly through software. These applications guide patients through treatment protocols for conditions such as mental health disorders, rehabilitation exercises, or chronic disease management.
By leveraging algorithms and user-inputted data, therapeutic applications ensure personalized care, enhancing patient outcomes and treatment adherence.
One of the coolest projects of this type that I’ve seen was a VR project for elderly people who deal with dementia. They had to put on VR headsets and solve various types of exercises helping to keep the mind in good shape or recover some capabilities. Digital health in the purest sense!
The rising popularity of Software as a Medical Device (SaMD) can be attributed to several key factors that align with current healthcare trends and technological advancements. These factors not only enhance patient care but also streamline processes within the medical sector.
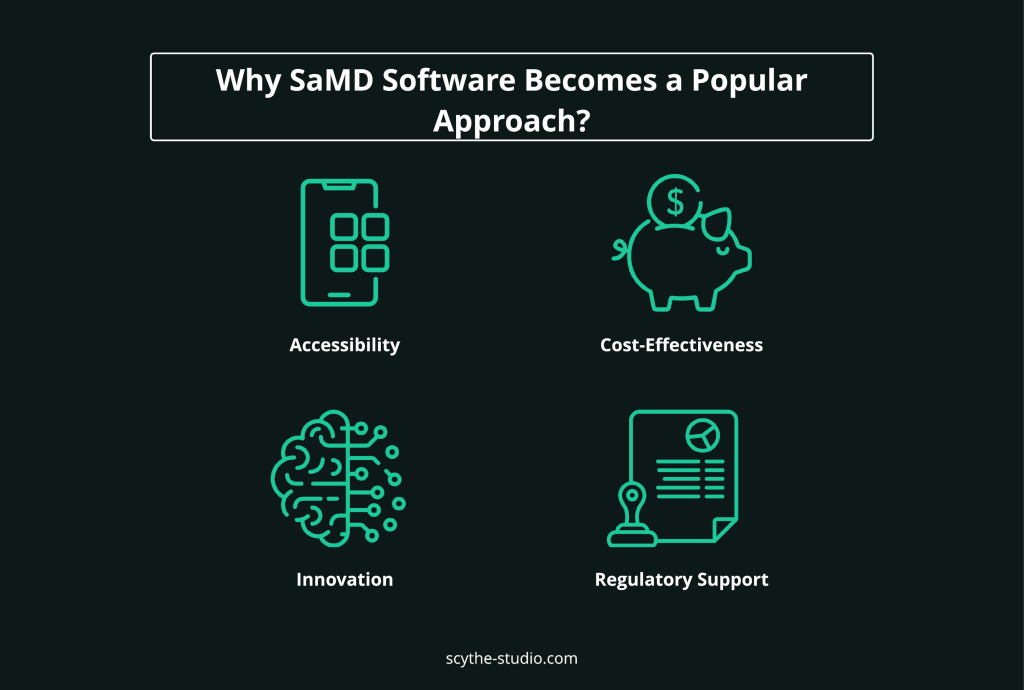
Software as a Medical Device applications offer unparalleled accessibility, allowing patients and healthcare providers to engage with medical tools and services from any location. This accessibility is especially beneficial in remote or underserved areas where traditional healthcare infrastructure may be lacking.
Additionally, the convenience of using SaMD software intended to run on personal devices like smartphones and computers fits seamlessly into daily life, encouraging more consistent and proactive healthcare management.
Implementing SaMD can significantly reduce costs for both healthcare providers and patients. By minimizing the need for physical infrastructure and in-person visits, these applications lower operational costs. They also expand the capacity of healthcare services without additional investments in physical resources.
Patients benefit from reduced travel expenses and time, which is particularly advantageous for those managing chronic conditions that require frequent monitoring and consultations.
The rapid pace of technological innovation in the fields of data analytics, artificial intelligence, and mobile technology has greatly enhanced the capabilities of SaMD. These technologies enable sophisticated diagnostic tools, personalized treatment plans, and real-time health monitoring, all within the same framework of regulatory compliance. By integrating cutting-edge technology, SaMD can offer more precise and effective medical solutions, advancing the overall standard of care.
Regulatory bodies have recognized the potential of SaMD and are actively updating guidelines to support its development and integration into healthcare systems. This evolving regulatory landscape ensures that SaMD products meet rigorous safety and efficacy standards, making them a trustworthy option for medical practitioners and patients alike.
The focus on regulatory compliance not only safeguards patient safety but also encourages innovation by providing clear frameworks for developers to follow. I have to say that Software as a Medical Device projects are not neglected here.
The combination of these factors explains why SaMD is becoming a preferred solution in the healthcare sector, promising a future where medical care is more personalized, accessible, and efficient.
Classifying Software as a Medical Device (SaMD) involves evaluating the software’s risk to patients and its functionality, following a risk-based approach set by regulatory bodies like the IMDRF. SaMD must comply with stringent standards for software quality, data security, and privacy. The classification determines the regulatory pathway, including necessary testing and potential clinical trials.
Later we will explain how the classification of SaMD projects differs from SiMD projects.
Speaking of the second type of medical software, you should imagine solutions that can’t fulfill their purpose without a dedicated physical medical device. Examples of SiMD systems can be sonographic instruments (even portable ones) or increasingly popular worldwide and extremely innovative systems for robotic surgery.
It is important to note here the sometimes subtle boundary between SiMD and SaMD. In some cases, there are problems with the proper classification of individual projects. For example, a phone app for taking glucose results from an external glucose meter or sensor attached to the body is not permanently “embedded” in a physical device, but requires it to carry out its purpose. It should therefore be considered part of the SiMD system.
Building SiMD projects may be an unbelievably costly undertaking. That’s why a lot of projects of this type, get financed externally and begin as regular startups.
Just like for software as a medical device, let’s take a look at some of the examples.
Surgical systems represent some of the most complex, time-intensive, and costly products for medical device manufacturers, and they are subject to stringent medical device regulatory processes. At Scythe Studio, we have been involved in numerous such projects.
One notable project we developed is a robotic-guided spine surgery system. In this system, a surgeon inputs medical images – CT or MRI scans of a patient’s spine into the software. Utilizing the software’s capabilities, the surgeon can meticulously plan the surgical procedure, including the placement of screws and trajectories.
Then robotic arms (parts of the system) are positioned accordingly, and the medical team carries out the operation as planned by the software. The goal is to ensure maximum precision.
The entire system was engineered using the C++ programming language and the Qt framework, enhanced by additional low-level technologies, and fully integrated with the necessary hardware medical device. This project stands as an example of cutting-edge technology applied to medical purposes.

Software that exclusively operates through interaction with a hardware medical device is also classified as Software in a Medical Device. A prime example of SiMD of this type is the egoo.health system in which we participated.
This system features a physical instrument that requires capsules filled with blood and a specific code to conduct biomarker analyses. The Egoo instrument, devoid of any controls like buttons or displays, necessitates the use of a mobile app to function effectively. This app connects to the instrument and the cloud platforms, enabling it to perform and manage the tests.
Since the software is intended to operate solely in conjunction with the hardware medical device, it exemplifies SiMD. For more insights into this project, visit Scythe Studio’s egoo.health case study.
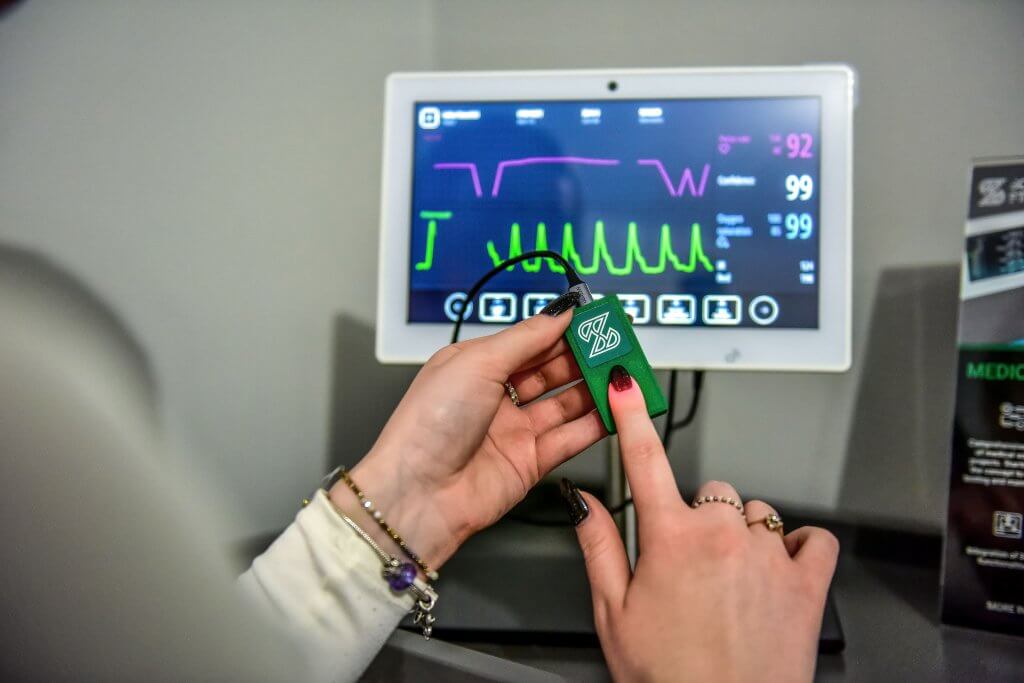
In another exemplary SiMD project, we developed a comprehensive patient monitoring system. This system, serving crucial medical equipment, integrates logic and user interface components alongside a newly created sensor prototype.
The project was designed for precision and ease of use. The software provides real-time monitoring of patient vitals, greatly simplifying the setup process for medical professionals. The aim is to assist healthcare practitioners in delivering high-quality care and enhancing patient outcomes.
The software, one of the core components of the proposed framework, operates on a specially designed medical-embedded tablet. Utilizing advanced programming languages like C++ and Qt framework, software developers at Scythe Studio crafted this innovative solution.
This project showcases the vital role of software in enhancing the functionality of medical devices.
Understanding the components that build medical devices is crucial in modern product development methodology, especially when distinguishing between Software in a Medical Device and Software as a Medical Device. For a deeper dive into the prototyping process of medical devices and the role of software, refer to this detailed article: Medical Device Prototypes and the Role of Software.
The foundation of any medical device is its hardware. Without the physical components, the functionalities necessary for medical purposes could not be realized.
Mechanical components are the bones of hardware medical devices, providing the structural integrity and movement capabilities necessary for their operation.
Electronics and sensors are critical for the functionality of medical devices, enabling the detection and measurement of patient data which are vital for health care.
Enclosures and casings protect the internal components of medical devices, ensuring durability and safety during operation, crucial for maintaining the integrity of patient data and device functionality.
Software plays a pivotal role in enhancing the capabilities of hardware medical devices, aimed at specific medical purposes.
Firmware is the low-level software that runs on embedded devices within the medical device. It is crucial for the basic operation and control of the device’s hardware, directly impacting its performance and reliability.
This high-level software provides user interaction interfaces for health care providers, often including critical features like DICOM integration for medical imaging purposes. It enhances the usability and functionality of the medical device, making it a vital component of digital health solutions.
On our blog you can find a great article “How to Choose the Right Technology for Health Care Software Development?“. We really can’t go that deep in deliberating on various software technologies here, so I recommend you add this one to your read list!
The remote site software extends the functionality of medical devices by enabling connectivity to various platforms such as PACS servers and computing platforms. This integration is essential for a comprehensive digital health ecosystem, allowing for more efficient management and analysis of patient data.
In this framework, both hardware and software components are interdependent, each adding layers of functionality and safety to the medical device. While hardware provides the necessary physical support and direct interaction with health care environments, software enriches these interactions.
The software here makes sophisticated medical purposes possible and differentiates SiMD from SaMD software, where the latter operates independently from physical devices. This synergy is essential for the development of effective medical instruments in today’s health care landscape.
In the realm of medical technology, distinguishing between Software as a Medical Device and Software in a Medical Device is crucial for healthcare industry professionals. Below is a concise overview of their key differences across various aspects:
SaMD: Operates independently from any hardware, fulfilling its intended medical functions through software alone, typically on general computing platforms.
SiMD: Integrates with other medical devices, requiring hardware to perform its functions. This software enhances or controls the hardware device’s operations.
SaMD: Projects can vary in scale but often focus solely on software development that can be used across multiple platforms.
SiMD: Typically involves larger, more complex projects as it integrates software with specific medical device hardware, often leading to advanced development challenges.
SaMD: Must comply with medical device regulation standards but is scrutinized primarily for its software capabilities and the direct health impact it delivers.
SiMD: Subject to stringent regulatory oversight not only for the software but also for how it interacts with the hardware components of the medical device.
SaMD: Development is agile, aimed at rapid deployment across various digital platforms. Updates and iterations are more straightforward given the software-centric nature.
SiMD: Development involves both software and hardware considerations, requiring a more integrated approach that often results in longer deployment times and complex update processes.
SaMD: Risk factors are generally evaluated based on the potential impact of the software’s decision-making on patient health.
SiMD: Risks are assessed not just on the software’s functionality but also on its integration with hardware, which can introduce additional risk factors.
Understanding these distinctions is vital for ensuring compliance with medical device regulations and effectively leveraging technology in healthcare settings. Both SaMD and SiMD play critical roles, yet they require different approaches to development, deployment, and oversight due to their inherent operational and regulatory differences.
Brief comparison of Software in Medical Device (SiMD) and Software as a Medical Device (SaMD) projects.
| SiMD | SaMD | |
|---|---|---|
| Functionality | Enhances hardware device functions | Operates independently as software |
| Project Complexity | Involves complex, integrated components. Potentially more expensive in development. | May range from small to large scale. Rather cheaper in the development |
| Development and Deployment | Requires long development and extensive integration testing | Smoother development process. Allows for quicker, more flexible updates |
| Regulatory Considerations | Subject to software and hardware regulations | Governed mainly by software regulations |
| Risk Classification | Risk assessed on software-hardware interaction | Risk based on software’s direct impact |
As digital health products evolve, there is a growing trend towards software-driven solutions, but will traditional medical devices be completely replaced by software equivalents? While software solutions such as SaMD are expanding and enhancing accessibility, offering better clinical management, they cannot fulfill all the functionalities of traditional hardware-based medical devices.
Traditional medical instruments often play complementary roles to software solutions. Certain clinical tasks require the precision of physical devices, especially in surgical interventions or where direct monitoring through embedded sensors is crucial.
However, the integration of software enhances these capabilities, enabling more refined control and sophisticated data analysis, leading to more accurate diagnoses and effective treatment plans.
Advancements in machine learning are propelling this shift towards software solutions. These technologies allow the software to learn from extensive collected data, enhancing decision-making and risk categorization. Machine learning is particularly effective in areas like diagnostic imaging and predictive analytics, where it can process and analyze data with a speed and accuracy that traditional methods may not achieve.
At Scythe Studio, we specialize in leveraging these advancements for medical device development, including Qt HMI development and custom software creation. If you’re looking to integrate the latest in machine learning into your medical device, contact us to discover how our expert services can elevate your product.
In summary, while software will not entirely replace traditional medical devices, its role is undeniably crucial in the ongoing transformation of healthcare. Together, hardware and software are set to deliver more comprehensive, accessible, and effective patient care, making them indispensable in the future of healthcare.
Let's face it? It is a challenge to get top Qt QML developers on board. Help yourself and start the collaboration with Scythe Studio - real experts in Qt C++ framework.
Discover our capabilities
Users of embedded devices – from industrial controllers to consumer electronics – are often unaware of hidden vulnerabilities that threaten […]

Graphical user interfaces (GUIs) are becoming more and more important in embedded devices – from home appliances to medical equipment […]
Technical managers in the embedded space often face a classic challenge: integrating industrial communication protocols into modern applications. One such […]