
Trading Software Development: A Comprehensive Guide
Trading software refers to the digital platforms and systems that enable investors and financial institutions to buy, sell, and manage […]
Join us at Qt C++ Warsaw Meetup - 21.08.2025
Sign up for free!
Medical software development has its own strict rules that must be followed. Because of how such software works, which, for example, may come into direct contact with patients or process patient data, such software must follow regulations to ensure safety, high quality, or performance.
In this post, I’ll outline the essential characteristics of the ISO 13485 standard. We’ll also discuss its role in medical software development and its benefits for manufacturers in the medical device industry. This article is not intended to be a tutorial for setting up a Quality Management System (QMS). It is more about introducing you, as a founder or director of a medical device company, to what ISO 13485 is in a nutshell.
I write this from the perspective of a B2B software company that builds software and hardware solutions for medical device companies. We successfully introduced a Quality Management System according to the ISO 13485 framework, so we know that, in addition to customer requirements, we have to meet regulatory requirements.
ISO 13485 influences our design and development services. Feel free to discover how Scythe Studio undertakes this challenge while providing medical device software development services.
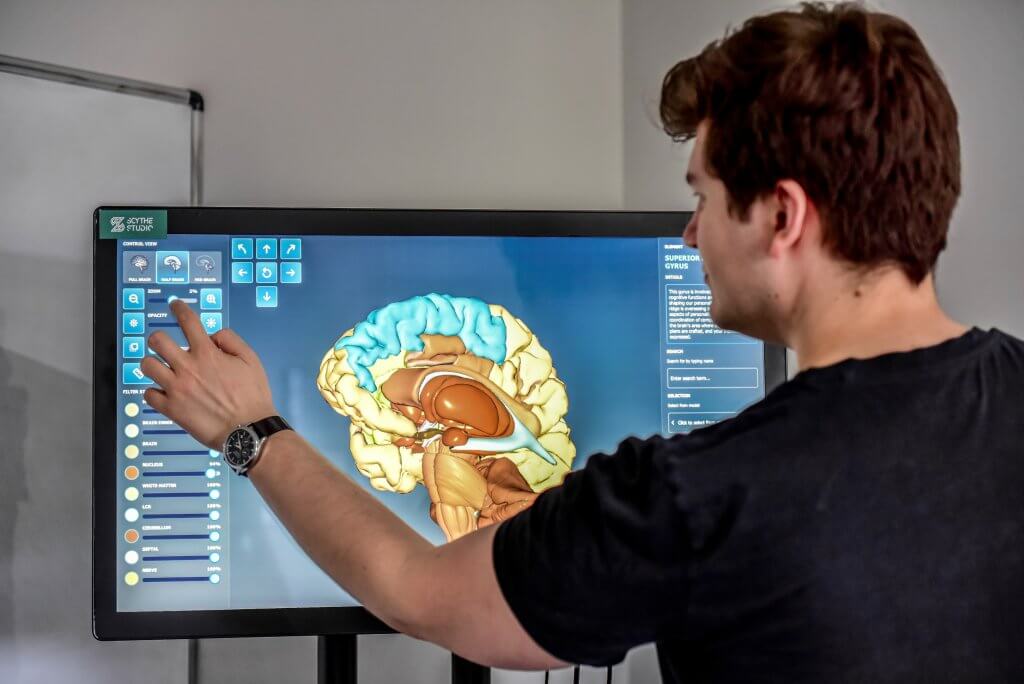 Cross-Platform Application for Previewing Parts of the Brain
Cross-Platform Application for Previewing Parts of the Brain
The ISO 13485:2016 standard is an international norm that has been developed for Quality Management Systems (QMS) for medical device manufacturing and designing. It defines principles that help medical device manufacturers meet end-user requirements and regulatory standards for safety and efficiency.
Setting up a compliant Quality Management System is a step that has to be made early in the development phase as medical device companies need it in place to comply with regulatory requirements and successfully submit their project. You should introduce QMS for both Software in Medical Device (SiMD) and Software as Medical Device (SaMD) projects. We described the differences between these two in one of our previous articles – SiMD vs SamD.
ISO 13485 is a standard developed by the International Organization for Standardization (ISO). It applies not only when a medical device is released to the market, but also throughout the entire life cycle of a medical device. Within this standard, there are requirements for risk management, quality management, and most important (to my mind) constant validation and improvement processes.
The standard itself follows many regulations in different parts of the world. For example, this norm is compatible with the CE mark in Europe, which indicates that a product has been tested by the manufacturer and meets EU requirements. Another example is the American FDA (Food and Drug Administration), which must provide a positive opinion of the safety and effectiveness of a medical product.
 ISO 13485
ISO 13485
You have to know that while ISO 13485 is a globally recognized standard, other medical organizations involved in regulatory compliance might have their own requirements for quality management. For example, the US. FDA (Food and Drug Administration) introduced 21 CFR 820.
Although 21 CFR 820 doesn’t directly incorporate ISO 13485 they are highly aligned. A lot of medical device companies start with a Quality Management System as the base and they then adjust some aspects of it to specific US. regulatory requirements.
21 CFR 820 was introduced to make it easier for companies to bring new devices to market. This is just my mischievous comment, but it is a pity that EU institutions do not come to similar conclusions.
 FDA 21 CFR 820
FDA 21 CFR 820
ISO 13485 is built around a Quality Management System (QMS), which helps companies organize and standardize how they develop and deliver medical devices. For medical software, a QMS means having processes to manage risks, document key actions, and check quality at each step. It’s not just about passing audits; it’s about building strong, repeatable processes that lead to safer products.
Medical device companies have to define their quality objectives and areas where Quality Management System is applicable. Those have to be activities directly impacting the development, quality, safety, and regulatory compliance of the medical devices. Remember that QMS doesn’t have to regulate all the operations at your organization.
Just to give you an example. For companies like Scythe Studio, product design and development activities like coding should be the subject of applicable regulatory requirements, but activities like HR, general administration, or marketing aren’t.
One of the main points of this certification is that management should play a key role in quality. Leaders must set quality goals, allocate resources, and ensure smooth communication across the team. This often means having leadership that fully supports quality, compliance, and risk management in medical software. When top management is committed to quality, it sets a solid foundation for everyone involved.
Working in the medical device industry, we learned that good examples and quality orientation should come from the top management.
 Quality Management System in medical industry
Quality Management System in medical industry
ISO 13485 highlights having the right resources in place, from qualified staff to a supportive work environment. Medical software requires skilled developers who also understand regulatory rules, so ongoing training and adequate infrastructure are essential. This focus on resources means that companies can maintain high standards and prepare teams to meet specific requirements in the medical field.
By resource management, I don’t mean only people, their competencies, and training, but also infrastructure, environment, and equipment. For example at Scythe Studio, conditions of work for software engineers are a subject of quality management. It is important to make sure that there is nothing that will interfere with proper design and development or bring cybersecurity issues.
Product realization covers every stage of bringing a product from an idea to a finished, market-ready product. For medical device software, this means creating software that works as promised, following strict steps for design, testing, and validation. ISO 13485, or more specifically good Quality Management System ensures each part of the product development is well-documented, so the final product is safe and effective for medical use. In this field, every detail matters, as patient safety relies on reliability and accuracy.
ISO 13485 also pushes for ongoing performance tracking and improvement. For medical software, this means regularly updating software, fixing bugs, and monitoring user feedback. By setting up regular checks and improvements, companies stay ahead of issues and keep products aligned with evolving medical needs. Continuous improvement is not just a nice to have, it’s essential for making sure the software remains safe and effective.
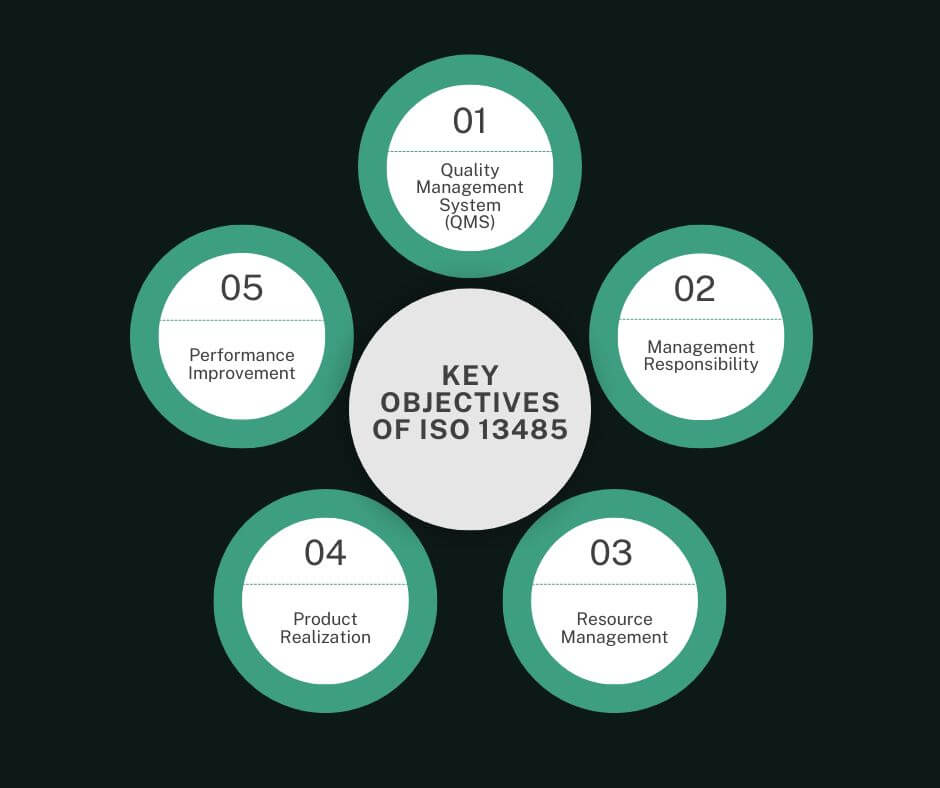 Key objectives of ISO 13485
Key objectives of ISO 13485
In medical device software development, ISO 13485 sets stringent requirements that directly impact software engineers, intertwining their work with robust Quality Management Systems (QMS). Engineers must follow structured processes for design, testing, and documentation, ensuring that each stage of development meets regulatory standards for safety and effectiveness. This means adhering to design controls, risk management practices, and traceability protocols that ISO 13485 mandates, all of which are essential for regulatory approval. By aligning their technical work with the QMS, software engineers play a critical role in safeguarding patient safety and compliance in the highly regulated medical device industry.
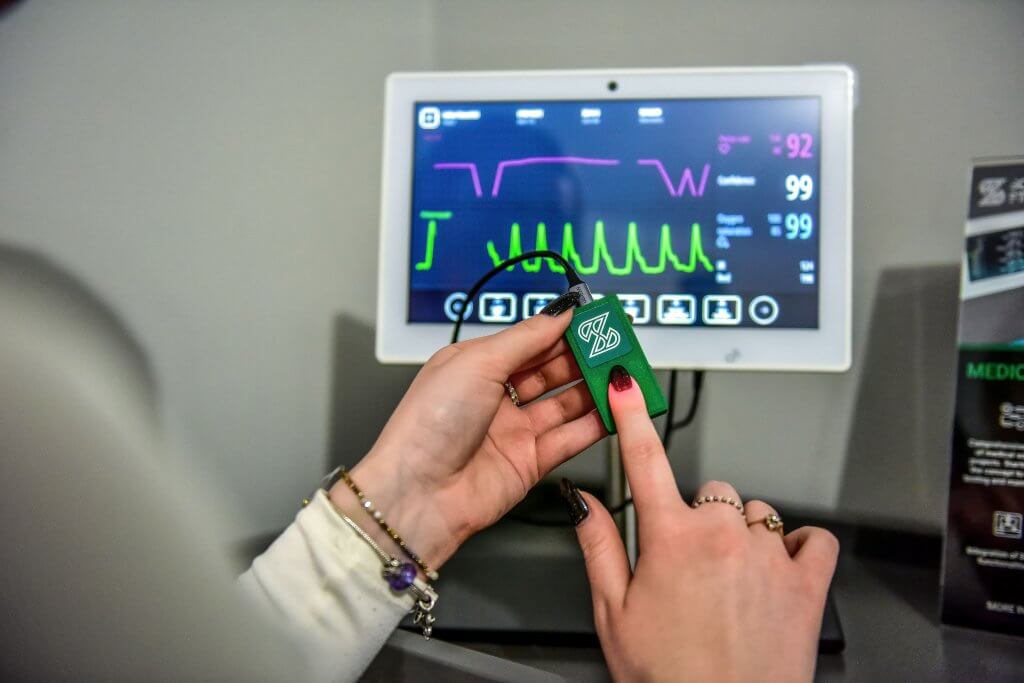 Medical device: Patient’s Vital Parameters Monitor
Medical device: Patient’s Vital Parameters Monitor
You have to know that there are more quality management standards out there. Both ISO 13485 and ISO 9001 deal with this topic. However, they have some crucial differences. ISO 9001 is a more general standard, focusing on customer satisfaction and continuous improvement across multiple sectors. It is applicable not only in the medical device industry.
On the other hand, ISO 13485, is specifically designed for medical devices, with an added emphasis on regulatory compliance, risk management, and detailed documentation. While ISO 9001 leans toward overall improvement, ISO 13485 prioritizes patient safety and regulatory requirements, which are most important in the medical device industry.
 ISO 13485 vs ISO 9001
ISO 13485 vs ISO 9001
Achieving ISO 13485 certification offers several advantages:
1. Regulatory compliance: ISO 13485 certification helps companies meet strict international standards and regulations for medical devices. With this certification, it’s easier to get products approved for different regions, saving time and reducing the risk of costly delays or rejections. It shows that a company’s products meet safety and effectiveness standards, which is essential for entering new markets.
2. Higher product quality: With ISO 13485 certification, companies follow structured quality management processes to design, develop, and manufacture medical devices. This organized approach leads to the development of reliable, safe, and effective products. As a result, companies can reduce the risk of failures, or safety issues, which can harm reputation and customer trust.
3. Market access: Many countries and healthcare markets require ISO 13485 certification to allow medical devices to be sold or used. With this certification, companies gain easier entry into international markets.
4. Increased Credibility: ISO 13485 certification demonstrates a company’s commitment to quality and safety. It sends a clear message to customers, patients, healthcare providers, and regulatory bodies that the company meets high standards.
In short, ISO 13485 certification isn’t just a regulatory requirement but also an asset that supports growth strengthens product quality, and builds a solid reputation in the medical device industry.
 Benefits of ISO 13485 certification
Benefits of ISO 13485 certification
Our path to achieving ISO 13485 certification compliance was a journey filled with both challenges and valuable lessons. This certification, which focuses on the quality management systems for medical devices, demanded a thorough look at every part of our operation. We had to dive deep into creating and maintaining detailed documentation, which required a lot of effort and attention to detail. It wasn’t just about writing things down; we had to ensure that all our records met specific regulatory standards.
Actually it was a great investment for us as it was the moment of serious improvement and many fruitful discussions about current and future processes that we have at the company.
One of the biggest challenges was making sure everyone on the team understood these standards and how they applied to their daily work. Regulatory guidelines can be complex, and translating them into practical actions was a learning curve for all of us.
We knew that for the certification process to succeed, every team member needed to be engaged and on the same page, so we prioritized training and open communication about the reasons for doing all of that.
 Scythe Studio’s ISO Certifications
Scythe Studio’s ISO Certifications
With a well-thought-out plan and a collaborative team spirit, we kept moving forward, facing each requirement step by step. Over time, this process didn’t just bring us closer to certification, it actually strengthened our internal systems.
Today, we’re seeing the benefits of our hard work. The certification has not only improved our focus on quality across medical device software development but also enhanced our ability to better understand customer requirements.
You can check out our recognitions on our certifications page.
 Scythe Studio’s ISO 13485 certificate
Scythe Studio’s ISO 13485 certificate
I wouldn’t say so. It may sound like a pile of unnecessary bureaucracy to those not involved in the medical device industry, but let’s remember that at the end of the day, it’s all about making sure no one gets hurt.
For Scythe Studio, implementing a QMS by ISO 13485 was key not only to show customers our commitment to quality but also to streamline the company’s operations. I’m pleased to say that this translates into customer satisfaction and thus the company. Feel free to contact us and discuss how we can support you in your software and hardware engineering.
Let's face it? It is a challenge to get top Qt QML developers on board. Help yourself and start the collaboration with Scythe Studio - real experts in Qt C++ framework.
Discover our capabilities
Trading software refers to the digital platforms and systems that enable investors and financial institutions to buy, sell, and manage […]

Verification and Validation (V&V) are two pillars that ensure a medical device is safe, effective, and compliant before it ever […]

From medical devices and smart vehicles to industrial controllers and consumer electronics, embedded systems are everywhere—and increasingly connected. But with […]